In Porous Media
1-Geochemical thermodynamics PHREEQC calculations
a) H2 Impact on a Well Cement :
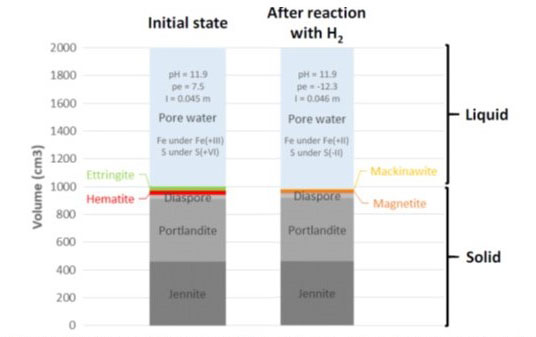
Representative Elementary Volume (REV) of the cement model before (left) and after (right) reaction with hydrogen. Boxes in grey nuances: non redox-sensitive minerals; coloured boxes: redox-sensitive minerals. Jennite: Ca9Si6H22O32; Portlandite: Ca(OH)2; Diaspore: AlO(OH); Hematite: Fe2O3; Ettringite: Ca6Al2(SO4)3(OH)12:26H2O; Magnetite: Fe3O4; Mackinawite: FeS. I: ionic strength; m: mol/kg of H2O. Fe(+III/+II): ferric/ferrous iron; S(+VI/-II): sulphates/sulphides. Porosity=[liquid volume (cm3)]/[REV (cm3)]; Initial porosity=1000/2000=50 %; Final (after reaction with hydrogen) porosity=1020/2000=51 %.
Main Reactions :
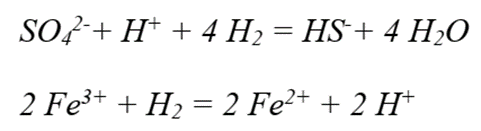
Sulfate and ferric iron reductions by hydrogen
b) H2 Impact on an Aquifer’s Reservoir Rock and on Water Quality :
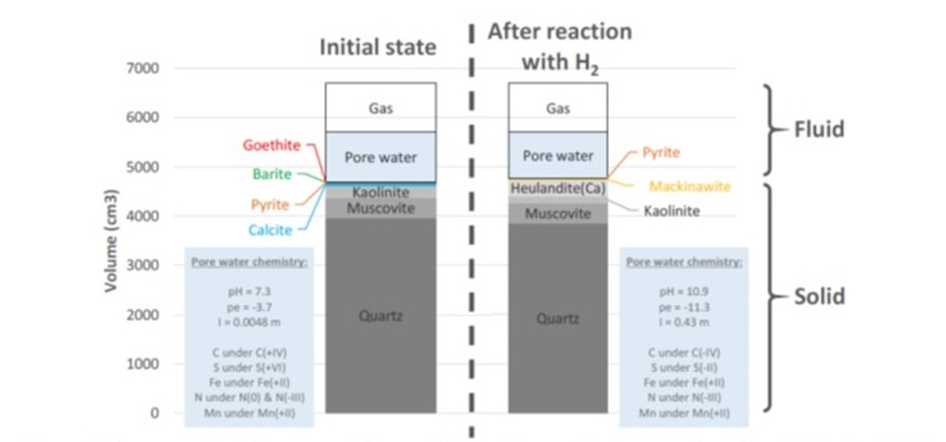
Representative Elementary Volume (REV) of the aquifer’s reservoir rock model before (left) and after (right) reaction with hydrogen. Boxes in grey nuances: non redox-sensitive minerals; coloured boxes: redox-sensitive minerals. See the text for the mineral formula. I: ionic strength. m: mol/kg of H2O. Porosity=[fluid volume (cm3)]/[REV (cm3)]; Initial porosity=2000/6700=30 %; Final (after reaction with hydrogen) porosity=1937/6700=29 %.
Main Reactions :
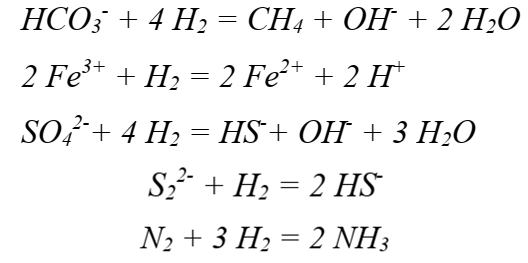
Methanogenesis (conversion of carbonates to methane) and ferric iron, sulftate and disulfide reductions by hydrogen
2-Experimental Work
Reactivity of a ferric iron oxides-bearing sandstone with hydrogen
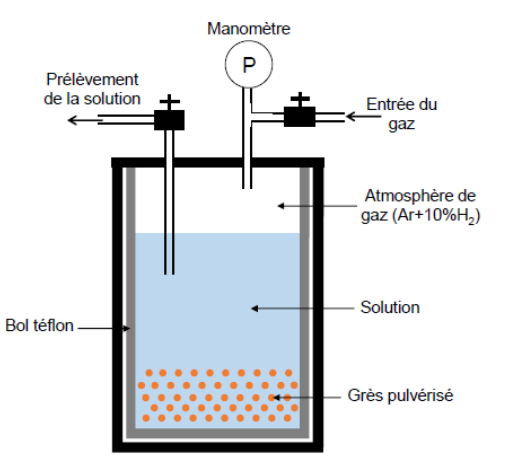
Scheme of a Parr reactor with an aqueous solution sampling device.

Grès broyé : avant expérience (a), après expérience en atmosphère H2+Ar (b),
après expérience en atmosphère Ar (c). La barre d’échelle représente 1 cm.
In Salt Caverns
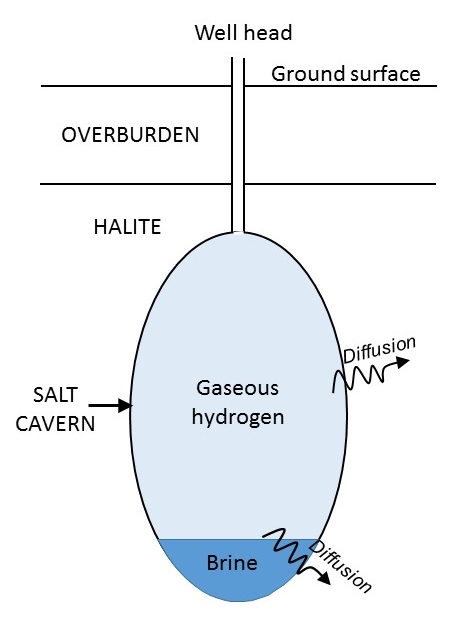
Hydrogen diffusion through salt & brine.
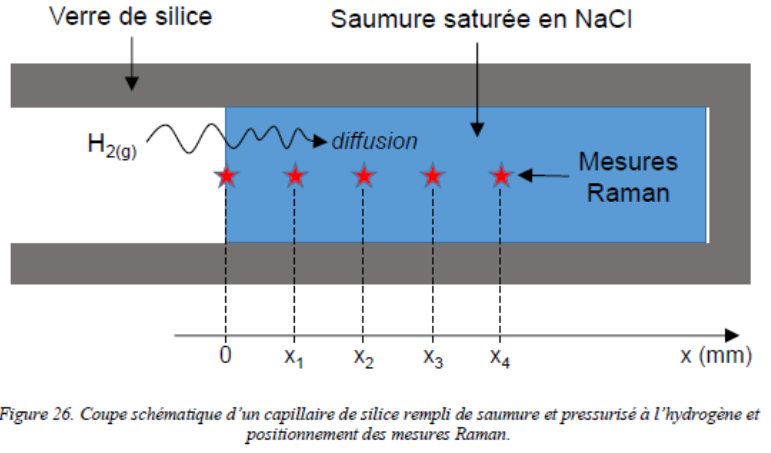
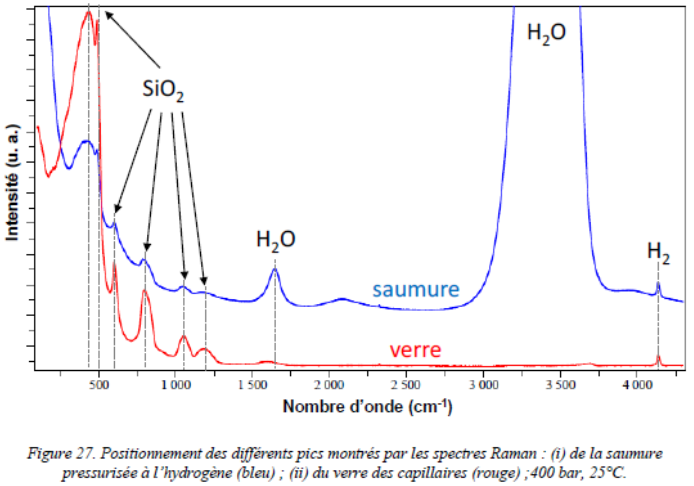
a) Hydrogen diffusion coefficient in halite mono-crystal determination by coupled Fused Silica Capillary Capsules (FSCCs) and Raman micro-spectroscopy experiments :
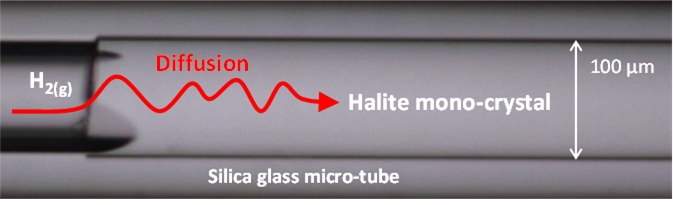
Halite micro-crystal face submitted to highly pressurized gaseous hydrogen in a silica glass capillary capsule.